#气锁间 #MAL物料气锁间
大家都知道,在生产车间设置物料气锁间(MAL)主要目的是在人员或物料出入其间时防止对高洁净室的污染。那么,它要如何进行合规管理呢?本期德小恩将通过一个相关话题,带大家深入了解气锁间的管理。
本期话题:在CNC与C级区之间的物料气锁间,如下图,从低级区域一侧传入物料后,没有建立缓冲间自净的要求(操作要求、时间),这是符合GMP的要求吗?
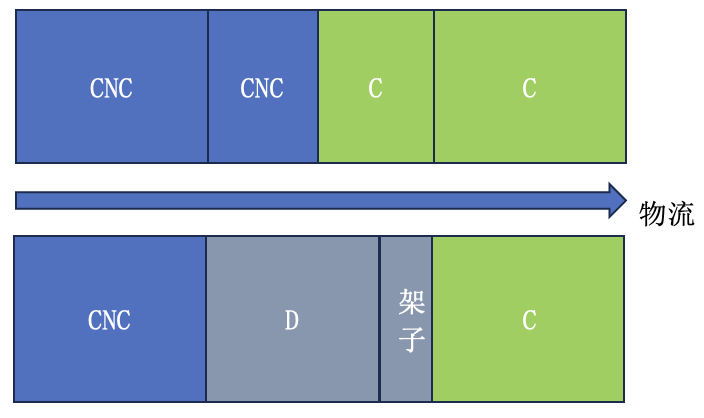
为此,德小恩对与气锁间相关的中美欧GMP法规中进行了总结,一起看看以上的问题是否符合气锁间的合规性管理呢?
相关法规要求
01 中国相关法规
1. GMP 2010版
第十四章 附则
(三十)气锁间
设置于两个或数个房间之间(如不同洁净度级别的房间之间)的具有两扇或多扇门的隔离空间。设置气锁间的目的是在人员或物料出入时,对气流进行控制。气锁间有人员气锁间和物料气锁间。
2. GMP2010版-无菌附录
第十条 应当按以下要求对洁净区的悬浮粒子进行动态监测:
(七)生产操作全部结束、操作人员撤出生产现场并经15~20分钟(指导值)自净后,洁净区的悬浮粒子应当达到表中的“静态”标准。
(八)应当按照质量风险管理的原则对C级洁净区和D级洁净区(必要时)进行动态监测。监控要求以及警戒限度和纠偏限度可根据操作的性质确定,但自净时间应当达到规定要求。
(九)应当根据产品及操作的性质制定温度、相对湿度等参数,这些参数不应对规定的洁净度造成不良影响。
第十二条 应当制定适当的悬浮粒子和微生物监测警戒限度和纠偏限度。操作规程中应当详细说明结果超标时需采取的纠偏措施。
第三十一条 气锁间两侧的门不得同时打开。可采用连锁系统或光学或(和)声学的报警系统防止两侧的门同时打开。
3. 2023版GMP指南 无菌制剂上册
a.一般控制区至C/D 级洁净区 (D 级洁净区至C 级洁净区)
物料向高洁净级别区域转移前,应首先被转移至物料气闸内,物料气闸通常作 为两个不同洁净级别房间之间的缓冲区域, 一般会配备连锁装置,可以是用于物料转移的房间,也可以是传递仓的形式。在缓冲气闸的设计上,应尽量使物流通道与人流通道分开,保证物料转移过程使用独立的物流通道,从而降低污染风险。不建议人流和物料共用通道,否则需保证人员更衣和物料转移操作不可同时进行,最大程度降低交叉污染风险。
在转移的过程中,企业应关注以下要点,以保证转移过程消毒的有效性:
气闸的设计应考虑连锁装置、压差控制、环境洁净级别等因素。
擦拭过程应保证物料表面全部被消毒剂润湿,特别注意容易被遮挡表面的消毒,如塑料袋接缝处等。
消毒剂接触时间(润湿时间)应经过验证并在实际操作中建立手段控制并监控是否达到规定的接触时间限度。
应建立标准的操作规程,详细规定转移和消毒的方法,保证操作过程的可重复性。
对于密封包装的无菌物料,在转移过程中应对其包装的完整性进行检查。
应关注消毒剂是否会对物品包装产生影响,进而影响物料的完整性和无菌性。
转移过程中建议尽量使用自动化的去污染设备(如蒸汽灭菌柜、 VHP 等)。如采用手工消毒,企业可考虑优先使用杀孢子剂,尽可能降低芽孢从低级别区域引入 高级别区域的风险,最大化降低微生物负荷和污染,保证消毒效果,如使用的杀孢 子剂存在残留,应在设计转移流程时考虑消毒剂去残留操作。
物料转移过程中,不同洁净级别区域工作、不同级别着装的操作者应避免同时出现在同一缓冲气闸。
如物料有多层包装,也可采用脱包装的方式代替消毒剂的消毒方式,但需要注意的是脱包装后物料表面的洁净级别,即物料对应包装层的初始包装环境,应满足转移后所在区域的环境洁净级别。
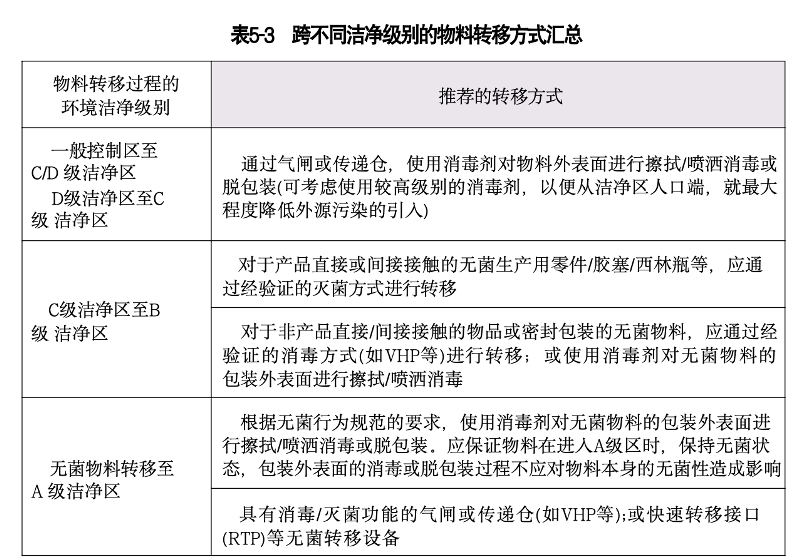
对于不能经过热力灭菌的物品,可以考虑其他合适的灭菌/去污染方式,如辐射、熏蒸、环氧乙烷灭菌、汽化过氧化氢(VHP) 去污染等方式,并在进入无菌区前,使用适当的消毒剂对物料包装外表面进行喷洒或擦拭消毒或紫外照射消毒后传人无菌室。例如环境监测碟传入B 级区流程可参考以下操作:首先将环境监测碟脱去物料最外层包装或对物料外包装进行清洁/消毒,然后放置于物料气锁间自净。自净结束后,从气锁间取出物料,转移到VHP 处理器内,按照经过验证的装载摆放 整 齐,经VHP 去污染后传入B级区(注:汽化过氧化氢表面处理是一种常见的生 物净化方式,相关内容可参考PDA 第34号报告)。
P.S. 本法规说明传入B级别前需要先到物料气锁间自净,而且传递方式最好为传递窗。
4. 2023版GMP指南 无菌制剂下
气锁设计
人员、设备和物料应通过气锁间进入洁净区
气锁的作用主要是维持压差以防止空气污染,有正压与负压两种类型,可用于人员进出及物料传递。气锁是两个不同洁净区间的连接通道,气锁应保持逐级压差梯度。气锁可采用连锁系统或光学和(或 )声学的报警系统防止两侧的门同时打开。
5. 2023版GMP指南-厂房设施与设备
3.5.2.4 气锁和缓冲
气锁室一般设在洁净室的出入口,是用以阻隔外界或邻室气流和进行压差控制所设置的缓冲间。为了尽量减小微粒传递速度(通常大于0.5m/s)所需的空气总量, 污染受控空间的所有门均应始终保持在关闭状态。若面积为2m2的区域的某扇门处于打开状态,则通过该门并包含微粒的空气流量可达约3500m3/h;若面积为2m2的区域的某扇门处于关闭状态,则仅会有160m3/h空气通过该门的裂缝漏出,用以阻止微粒进入。
P.S. 开关门对环境的影响:开门后,通过压差梯度去说明被污染风险低是不科学的。
在负压气锁室中,虽然排出的空气量多于送风量,仍建议向该区域多送些风, 以使气锁室能更快地从被污染状态恢复过来。
为确保负压,各门的操作模式应让其保持在关闭状态。建议气锁室配备联锁系统或视觉、音响报警系统,用以防止一次打开一扇以上的门。处于关闭状态的门仅能为微粒提供很小的通过面积,故仅需较小的气流即可防止微粒进入。
02 EU相关法规
1. Annex 1 Manufacture of Sterile Medicinal Products
4 Premises
4.1 The manufacture of sterile products should be carried out in appropriate cleanrooms, entry to which should be through change rooms that act as airlocks for personnel and airlocks for equipment and materials. Cleanrooms and change rooms should be maintained to an appropriate cleanliness standard and supplied with air that has passed through filters of an appropriate efficiency. Controls and monitoring should be scientifically justified and should effectively evaluate the state of environmental conditions of cleanrooms, airlocks and pass-through hatches.
P.S. 本章节表明需要设计气锁间,人和物料不可共用通道。
4.12 Airlocks should be designed and used to provide physical separation and to minimize microbial and particle contamination of the different areas and should be present for material and personnel moving between different grades. Wherever possible, airlocks used for personnel movement should be separated from those used for material movement. Where this is not practical, time-based separation of movement (personnel / material) by procedure should be considered. Airlocks should be flushed effectively with filtered air to ensure that the grade of the cleanroom is maintained. The final stage of the airlock should, in the “at rest” state, be of the same cleanliness grade (viable and total particle) as the cleanroom into which it leads. The use of separate change rooms for entering and leaving the grade B area is desirable. Where this is not practical, time-based separation of activities (ingress/egress) by procedure should be considered. Where the CCS indicates that the risk of contamination is high, separate change rooms for entering and leaving production areas should be used. Airlocks should be designed as follows:
II. Material airlocks: used for materials and equipment transfer.
Only materials and equipment that have been included on an approved list and assessed during validation of the transfer process should be transferred into the grade A or grade B areas via an airlock or pass-through hatches. Equipment and materials (intended for use in the grade A area) should be protected when transiting through the grade B area. Any unapproved items that require transfer should be pre-approved as an exception. Appropriate risk assessment and mitigation measures should be applied and recorded as per the manufacturer's CCS and should include a specific disinfection and monitoring programme approved by quality assurance.
Pass-through hatches should be designed to protect the higher-grade environment, for example by effective flushing with an active filtered air supply.
The movement of material or equipment from lower grade or unclassified area to higher-grade clean areas should be subject to cleaning and disinfection commensurate with the risk and in line with the CCS.
P.S. 气锁缓冲间,洁净度不能低于高级别的洁净区
4.29 Cleanroom classification should be carried out in the “at rest” and “in operation” states.
III.The total particle limits given in Table 1 above for the “at rest” state should be achieved after a “clean up” period on completion of operations and line clearance/cleaning activities. The "clean up" period (guidance value of less than 20 minutes) should be determined during the qualification of the rooms, documented and adhered to in procedures to reinstate a qualified state of cleanliness if disrupted during operation.
03 US相关法规
1. Sterile Drug Products Produced by Aseptic Processing — CGMP
Due to the interdependence of the various rooms that make up an aseptic processing facility, it is essential to carefully define and control the dynamic interactions permitted between cleanrooms. Use of a double-door or integrated sterilizer helps ensure direct product flow, often from a lower to a higher classified area. Airlocks and interlocking doors will facilitate better control of air balance throughout the aseptic processing facility. Airlocks should be installed between the aseptic manufacturing area entrance and the adjoining unclassified area. Other interfaces such as personnel transitions or material staging areas are appropriate locations for air locks. It is critical to adequately control material (e.g., in-process supplies, equipment, utensils) as it transfers from lesser to higher classified clean areas to prevent the influx of contaminants. For example, written procedures should address how materials are to be introduced into the aseptic processing room to ensure that room conditions remain uncompromised. In this regard, materials should be disinfected according to appropriate procedures or, when used in critical areas, rendered sterile by a suitable method.
04 其它法规相关要求
1. ISO 14644-4
B.3.5 Particle removal rate
The ventilation performance of a cleanroom can be expressed by its ability to reduce the particle concentration when there is an increased particle concentration from a particle source for a short time. In addition, airlocks need to be designed to ensure that the airborne particles will be removed to an acceptable low concentration before the door is opened. It can also be necessary to determine the time required to wait for the normal supply air volume flow rate to a cleanroom to re-establish the correct operational conditions from a 'turn-down' condition. Finally, it might be necessary to obtain the ventilation effectiveness at a critical location by measuring the recovery rate at the critical location and then comparing it with the overall air change rate of the cleanroom so as to obtain the ACE index. All of these four requirements involve the application of the removal rate or recovery time methods suggested in ISO 14644-3.
B.6.2 Airlocks
To minimize the transfer of contamination between rooms of different cleanliness classes during personnel and material transfer, a physical segregation by means of an airlock and/or pass-through box should be provided. The airlock and/or pass-through box should also maintain the pressure differential established between the rooms by ensuring the opposing doors are not opened simultaneously. For airlock types and selection see Reference.
Any airborne contamination released in the airlock and/or pass-through box should be contained and removed by a combination of interlocked doors and dilution by clean supply air for a defined flushing time. The effectiveness of air locks is dependent on the amount of air supplied and the time used to clear particle contamination. Information required to calculate these two variables is given in B.3.5. An airlock can have a higher air change rate than the cleanroom, to reduce recovery time and avoid contamination of the cleanroom upon entry.
Precautions should be taken to ensure that entry and exit doors associated with an airlock and/or pass- through box are not opened simultaneously. Clear windows can be provided at both points to allow a line-of-sight view. Consideration should be given to the use of electrical or mechanical interlock systems, including audio-visual indicators.
Stepover benches or other clear demarcation systems should be included within an airlock system for the passage of personnel.
总结:
1. 从低级区域一侧传入物料后,没有建立缓冲间自净的要求(操作要求、时间),是否可以?对此各国均没有直接的法规要求,只规定CNC→D/C的物流气锁需要自净时间;欧美法规有原则性的要求,不能污染高洁净区一侧;ISO中明确提及的关键点:
a) 设计需要满足要求(制药行业对应地需要验证:空调系统自净验证/工艺验证/气锁间验证);
b) 自净时间计算方法;
P.S. 本文章为德恩原创,如需转载,请注明来源于德恩GMP咨询。